Discussion
Discussion
FAQs about Soliris
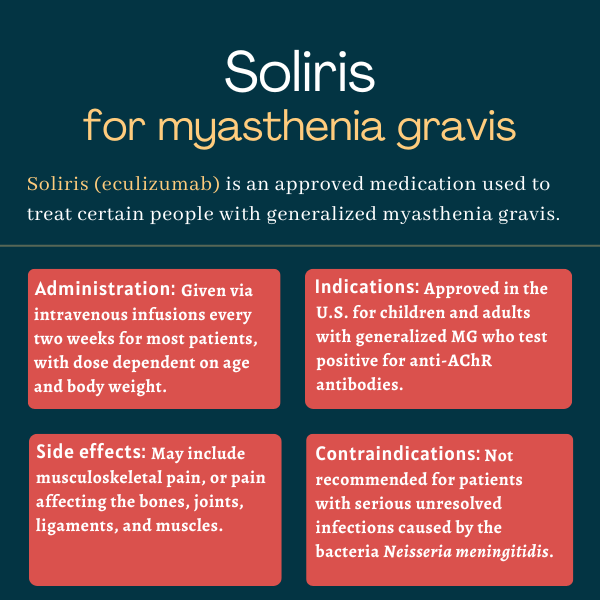
The U.S. Food and Drug Administration (FDA) initially approved Soliris in 2017 to treat adults with generalized myasthenia gravis (gMG) who are positive for antibodies against the acetylcholine receptor (AChR). That marked the first approval for the disease in the U.S. in more than 60 years. In 2025, the FDA approved a label expansion, extending the use of Soliris to children with AChR-positive gMG, ages 6 and older. This made Soliris the first and only therapy to be approved for pediatric gMG patients in the U.S.
In the REGAIN trial, which supported Soliris’ approval for myasthenia gravis, the majority of patients responded to treatment and experienced clinically meaningful improvements in the first three months. However, each patient is unique and may respond differently to the medication. A discussion with a healthcare provider can help patients understand how Soliris may help in their specific case.
Changes in weight and hair loss have not been reported in clinical trials testing Soliris in people with generalized myasthenia gravis. However, some patients receiving the medication in other clinical trials have reported hair loss. Patients who experience unusual symptoms while on treatment should discuss these with their care team.
While animal data has suggested that anti-C5 antibodies like Soliris can cause fetal harm, an analysis involving more than 300 women exposed to Soliris during pregnancy has not identified any major concerns. Still, it remains unknown if Soliris can cause harm to a developing human fetus, so patients who become or plan to become pregnant while on the medication should discuss this topic with their healthcare team.
Soliris has little to no impact on a person’s ability to drive and use machines. Yet, patients should be careful when attempting to drive or use machines before they know exactly how the medication affects them. Those taking Soliris should speak with their healthcare providers to know more about how the treatment could impact their ability to drive.
 Fact-checked by
Fact-checked by