Uplizna (inebilizumab-cdon) for myasthenia gravis
What is Uplizna for myasthenia gravis?
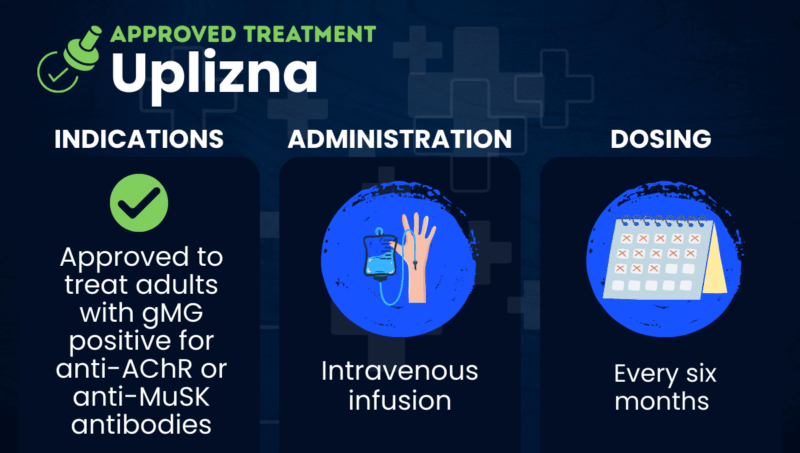
Uplizna (inebilizumab-cdon) is an antibody-based therapy approved for adults with generalized myasthenia gravis (gMG) who are positive for antibodies targeting the acetylcholine receptor (AChR) or muscle-specific tyrosine kinase (MuSK) proteins.
Myasthenia gravis (MG) is an autoimmune condition driven by self-reactive antibodies that mistakenly attack proteins involved in nerve-muscle communication — most often, AChR or MuSK. This leads to the disease’s hallmark symptoms of muscle weakness and fatigue. In gMG, these symptoms can affect several muscle groups throughout the body.
Uplizna is designed to deplete, or lower the number of B-cells — the immune cells mainly responsible for producing antibodies, including those that drive MG. The antibody-based therapy binds to CD19, a protein found on the surface of B-cells, presumably triggering an immune mechanism that leads to their destruction. In doing so, the medication is expected to reduce the levels of MG-causing antibodies, thereby easing disease activity and symptoms.
Administered via intravenous, or into-the-vein, infusion, Uplizna is also approved to treat two other B-cell-driven conditions: neuromyelitis optica spectrum disorder and immunoglobulin G4-related disease.
The therapy is marketed by Amgen.
Therapy snapshot
| Brand name | Uplizna |
| Chemical name | Inebilizumab-cdon |
| Usage | Used to treat adults with gMG who are positive for antibodies targeting AChR or MuSK |
| Administration | Intravenous infusion |
Who with myasthenia gravis can take Uplizna?
In the U.S., Uplizna is approved to treat adults with gMG who test positive for antibodies targeting AChR or MuSK.
The therapy is contraindicated, or should not be used, by people who:
- previously had a life-threatening infusion reaction to the medication
- have an active hepatitis B infection
- have active or untreated latent tuberculosis
How is Uplizna administered in myasthenia gravis?
Uplizna is administered as an intravenous infusion under the close supervision of an experienced healthcare professional. Each infusion lasts about 90 minutes. Patients will be monitored for infusion-related reactions during and for at least one hour after receiving Uplizna.
The recommended dosage is 300 mg. The first two doses are given two weeks apart, with subsequent doses being given every six months.
Before every infusion, patients will be evaluated for the presence of active infections. If an infection is present, treatment with Uplizna should be delayed until it resolves. Patients may also be given additional medications, including anti-inflammatory, anti-allergy, and fever-reducing medications, to reduce the risk of infusion-related reactions.
Uplizna in myasthenia gravis clinical trials
Uplizna’s approval for gMG was based on data from the placebo-controlled Phase 3 MINT trial (NCT04524273), which enrolled 238 adults with gMG who had anti-AChR or anti-MuSK antibodies. Results showed that:
- After 26 weeks (around six months), Uplizna led to a statistically significant reduction (improvement) in the scores of the MG Activities of Daily Living (MG-ADL), a patient-reported scale of MG severity, in the overall population of patients compared with a placebo. Statistically significant improvements were also seen in the scores of the Quantitative MG (QMG), a clinician-rated scale of muscle weakness.
- Improvements in MG-ADL scores were maintained for a year in the subset of patients who had anti-AChR antibodies. During this time, more than 70% of these patients saw their MG-ADL scores drop by at least three points, a milestone that was achieved by less than half of those given a placebo.
Amgen is also sponsoring a Phase 2 study (NCT06987539), which is testing Uplizna in children ages 2 to 17 with gMG. That study is expected to conclude in 2030.
Uplizna side effects
The most common side effects of Uplizna for people with gMG are headaches and infusion-related reactions.
Uplizna also comes with warnings for potentially serious side effects, including:
- infusion-related reactions, including life-threatening allergic reactions (anaphylaxis)
- increased susceptibility to infections, including serious, life-threatening, or fatal infections
- reduction in antibody (immunoglobulin) levels
- fetal harm
Uplizna should be permanently discontinued if a life-threatening or disabling infusion reaction occurs. Its administration should also be delayed in patients with an active infection until it resolves. The therapy may also have to be discontinued in patients with low immunoglobulin levels who develop a serious opportunistic infection or recurrent infections.
Vaccination with live-attenuated or live vaccines is not recommended during treatment with Uplizna and after discontinuation until B-cell numbers recover. For this reason, these vaccines should be administered at least a month before treatment initiation.
Animal data have indicated that Uplizna can cause fetal harm. Female patients of reproductive age should be informed of the potential risks to a fetus and use an effective method of contraception during treatment and for six months after stopping Uplizna.
Myasthenia Gravis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by